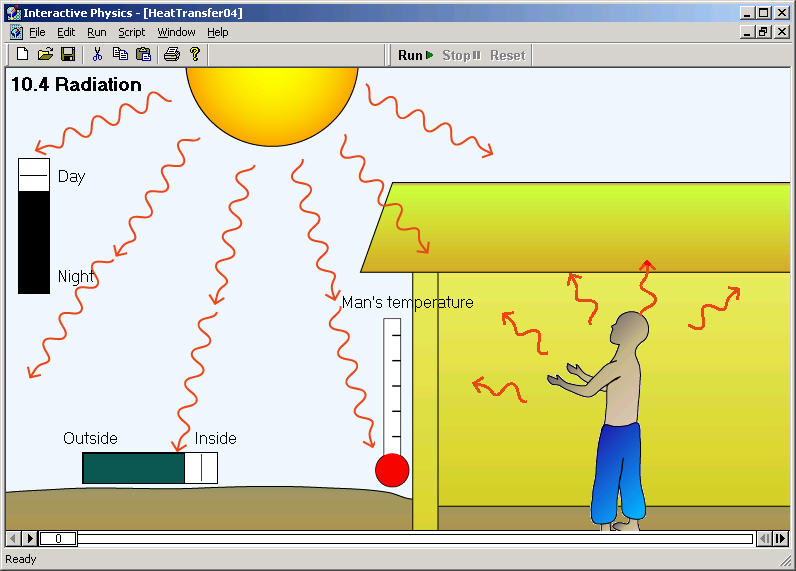
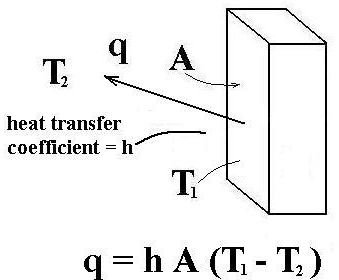
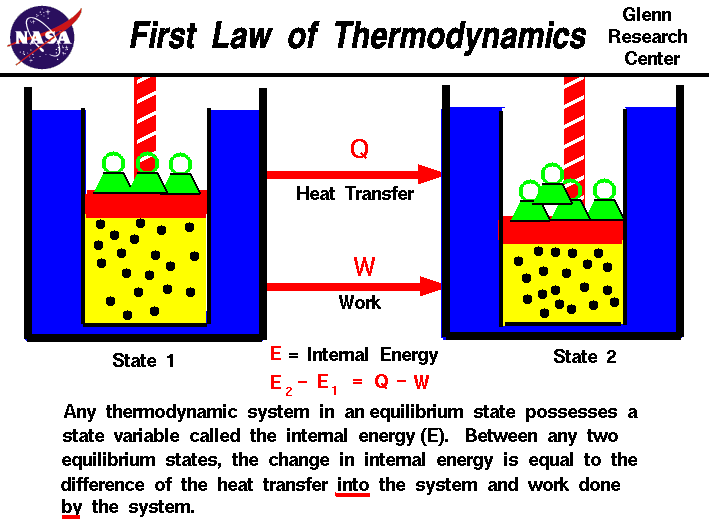
Turbo machinery is mainly of two types, one that add energy (Pumps), and other that extract energy (Turbines). The prefix turbo- is a Latin word which means “spin’’ or “whirl,’’.
The pump is the oldest fluid-energy-transfer device known. At least two designs
date before Christ: (1) the undershot-bucket waterwheels, or norias, used in Asia and Africa (1000 B.C.) and (2) Archimedes’ screw pump (250 B.C.), still being manufactured today to handle solid-liquid mixtures. Paddlewheel turbines were used by the Romans in 70 B.C., and Babylonian windmills date back to 700 B.C.
Machines which deliver liquids are simply called pumps, but if gases are involved,
three different terms are in use, depending upon the pressure rise achieved. If the pressure rise is very small (a few inches of water), a gas pump is called a fan; up to 1 atm, it is usually called a blower; and above 1 atm it is commonly termed a compressor.
The pump is the oldest fluid-energy-transfer device known. At least two designs
date before Christ: (1) the undershot-bucket waterwheels, or norias, used in Asia and Africa (1000 B.C.) and (2) Archimedes’ screw pump (250 B.C.), still being manufactured today to handle solid-liquid mixtures. Paddlewheel turbines were used by the Romans in 70 B.C., and Babylonian windmills date back to 700 B.C.
Machines which deliver liquids are simply called pumps, but if gases are involved,
three different terms are in use, depending upon the pressure rise achieved. If the pressure rise is very small (a few inches of water), a gas pump is called a fan; up to 1 atm, it is usually called a blower; and above 1 atm it is commonly termed a compressor.