The First Law of Thermodynamics states:
"Energy can neither be created nor destroyed, only altered in form".
For any system, energy transfer is associated with mass and energy crossing the control boundary, external work and/or heat crossing the boundary, and the change of stored energy within the control volume. The mass flow of fluid is associated with the kinetic, potential, internal, and "flow" energies that affect the overall energy balance of the system. The exchange of external work and/or heat complete the energy balance.
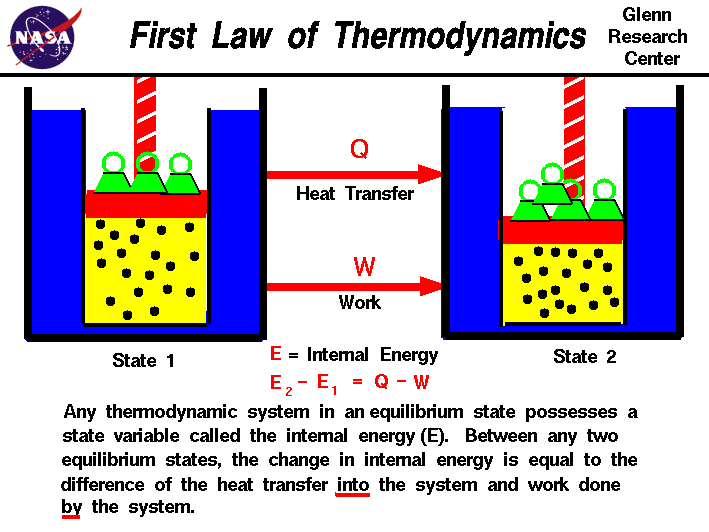
The First Law of Thermodynamics is referred to as the Conservation of Energy principle, meaning that energy can neither be created nor destroyed, but rather transformed into various forms as the fluid within the control volume is being studied. The energy balance spoken of here is maintained within the system being studied. The system is a region in space (control volume) through which the fluid passes. The various energies associated with the fluid are then observed as they cross the boundaries of the system and the balance is made.
"Energy can neither be created nor destroyed, only altered in form".
For any system, energy transfer is associated with mass and energy crossing the control boundary, external work and/or heat crossing the boundary, and the change of stored energy within the control volume. The mass flow of fluid is associated with the kinetic, potential, internal, and "flow" energies that affect the overall energy balance of the system. The exchange of external work and/or heat complete the energy balance.

The First Law of Thermodynamics is referred to as the Conservation of Energy principle, meaning that energy can neither be created nor destroyed, but rather transformed into various forms as the fluid within the control volume is being studied. The energy balance spoken of here is maintained within the system being studied. The system is a region in space (control volume) through which the fluid passes. The various energies associated with the fluid are then observed as they cross the boundaries of the system and the balance is made.









0 comments:
Post a Comment